Chapter 7 Review Chemical Formulas And Chemical Compounds
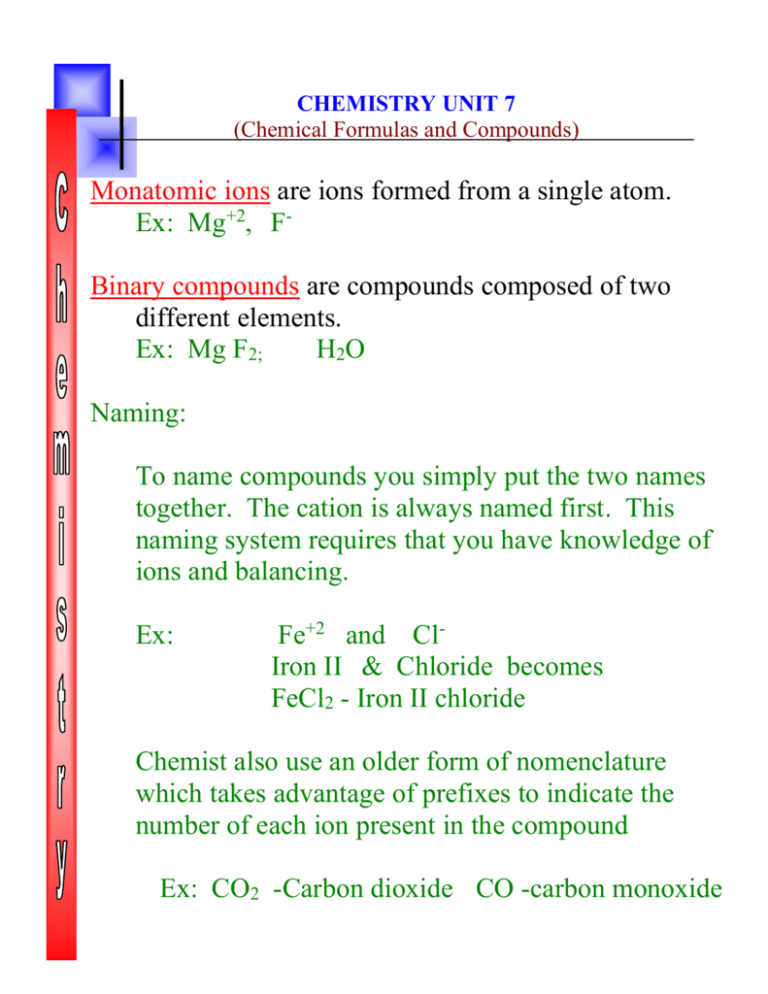
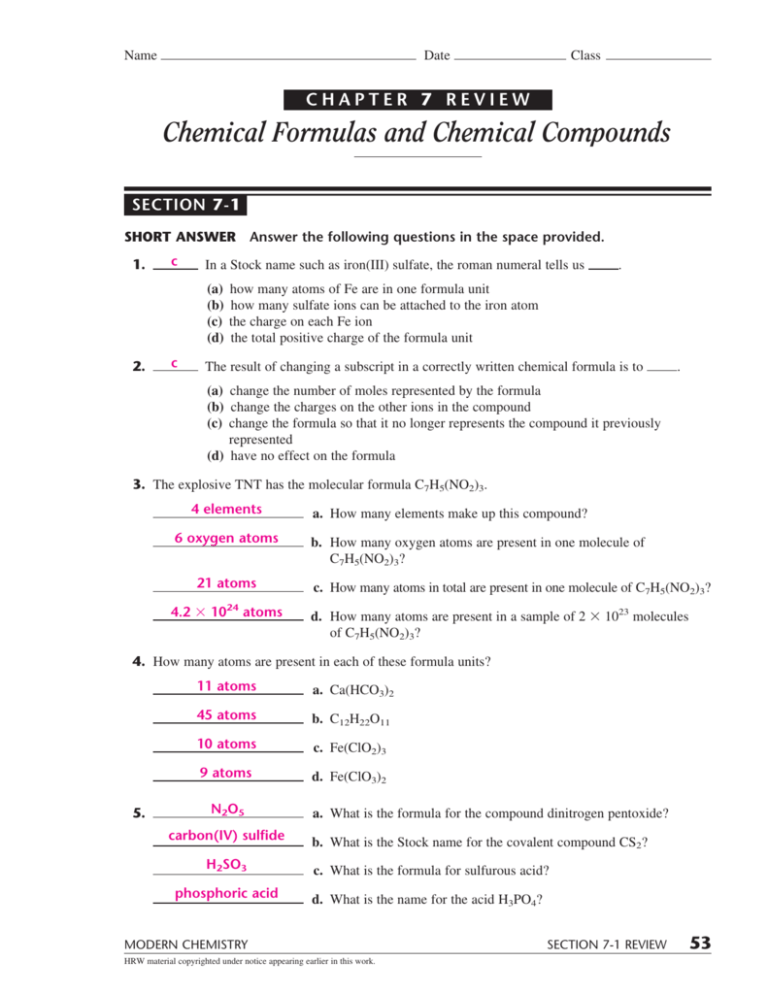
Chapter 7 Review Chemical Formulas And Chemical Compounds - Web benzene, chemical compound, carbonyl compounds, carboxylic acids, acyl compounds, chemical bonding, chemistry of life, electrode potential, electrons in atoms, enthalpy change, equilibrium, group iv, groups ii and vii, halogenoalkanes, hydrocarbons, introduction to organic chemistry, ionic equilibria, lattice energy, Label each of the following statements as true or false: The atoms in a pure element have an oxidation number of zero. Chemical formulas and chemical compounds. Web chapter 7 objectives •explainthe significance of a chemical formula. _____ in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula unit. Web chapter 7 review : Web a) charge on an ion. If the formula mass of one molecule is x u, the. Click the card to flip 👆. Web short answer answer the following questions in the space provided. •namean ionic compound given its formula. Web benzene, chemical compound, carbonyl compounds, carboxylic acids, acyl compounds, chemical bonding, chemistry of life, electrode potential, electrons in atoms, enthalpy change, equilibrium, group iv, groups ii and vii, halogenoalkanes, hydrocarbons, introduction to organic chemistry, ionic equilibria, lattice energy, •writethe formula of a. Focus on this content, but make sure to review class notes, activities, handouts, questions, etc. Some of the group 2 metals react with oxygen to form peroxides. •using prefixes, namea binary molecular compound from its formula. Chemical formulas and chemical compounds. Web chapter 7 chemical formulas and chemical compounds the following pages contain the bulk (but not all) of the. Chemical formulas and chemical compounds (mixed review) copper (ii) carbonate click the card to flip 👆 cuco₃ click the card to flip 👆 1 / 28 flashcards test created by blonde10123 terms in this set (28) copper (ii) carbonate cuco₃ sodium sulfite na₂so₃ ammonium phosphate (nh₄)₃po₄ tin (iv) sulfide sns₂ nitrous acid hno₂ The atoms in a pure element have. Write the formulas for these compounds. Web short answer answer the following questions in the space provided. Web modern chemistry 61 chemical bonding chapter 7 review chemical formulas and chemical compounds mixed review short answer answer the following questions in the space provided. Web chapter 7 review. Web chemistry chapter 7 review (names and formulas) term. Web short answer answer the following questions in the space provided. (d) the total positive charge of the formula unit. _____ changing a subscript in a correctly written chemical formula If the formula mass of one molecule is x u, the. Write formulas for the following compounds: If the formula mass of one molecule is x u, the. (d) the total positive charge of the formula unit. Write the stock system names for the following compounds: Web benzene, chemical compound, carbonyl compounds, carboxylic acids, acyl compounds, chemical bonding, chemistry of life, electrode potential, electrons in atoms, enthalpy change, equilibrium, group iv, groups ii and vii, halogenoalkanes, hydrocarbons,. (b) how many sulfate ions can be attached to the iron atom. Web compounds composed of two elements. Common chemicals and their common names naming ionic and covalent compounds oxidation numbers oxidation numbers #2 valence electrons and oxidation state oxidation numbers molecular and empirical formulas use this flowchart for naming compounds. Ionic compounds are composed to elements in an _______________. C) type of bond holding particles together in a compound. Focus on this content, but make sure to review class notes, activities, handouts, questions, etc. The percentage composition of sulfur in so2 is about 50%. Web modern chemistry 61 chemical bonding chapter 7 review chemical formulas and chemical compounds mixed review short answer answer the following questions in the space. Write formulas for the following compounds: Label each of the following statements as true or false: Their chemical formula reveals the number of atoms of each element in a single molecule of the compound. Write the formulas for these compounds. Chapter 5 the legislative branch 41 terms. C) type of bond holding particles together in a compound. D) distribution of electrons among the bonded particles in a compound. Chemical formulas and chemical compounds. Click the card to flip 👆. Ionic compounds are composed to elements in an _______________ bond. Some of the group 2 metals react with oxygen to form peroxides. Web modern chemistry 61 chemical bonding chapter 7 review chemical formulas and chemical compounds mixed review short answer answer the following questions in the space provided. Atoms have an oxidation number of zero in a (n) a) pure element. Web answer the following questions in the space provided. (c) the charge on each fe ion. (d) the total positive charge of the formula unit. Oxidation numbers are assigned to the atoms composing a compound or ion in order to indicate the general distribution of electrons among the bonded atoms in the compound or ion. _____ changing a subscript in a correctly written chemical formula If you study this document and nothing else, you should at least be able to pass the test. Study with quizlet and memorize flashcards containing terms like the _____ _______ contains all the elements, arranged according to similarities in their properties., the ________ are on the right hand side of the. Study with quizlet and memorize flashcards containing terms like hydrocarbons, monatomic ions, stock system and more. Web a) charge on an ion. (d) the total positive charge of the formula unit. Chapter 4 the bill of rights 33 terms. (b) how many sulfate ions can be attached to the iron atom. Write formulas for the following compounds: Write the stock system names for the following compounds: Chemical compounds and chemical formulas chapter interactives: The empirical formula for a compound shows the symbols of the elements with subscripts indicating the. Web chapter 7 review :Chapter 7 Chemical Formulas And Chemical Compounds Answer Key
Chapter 7 Formulas & Compounds
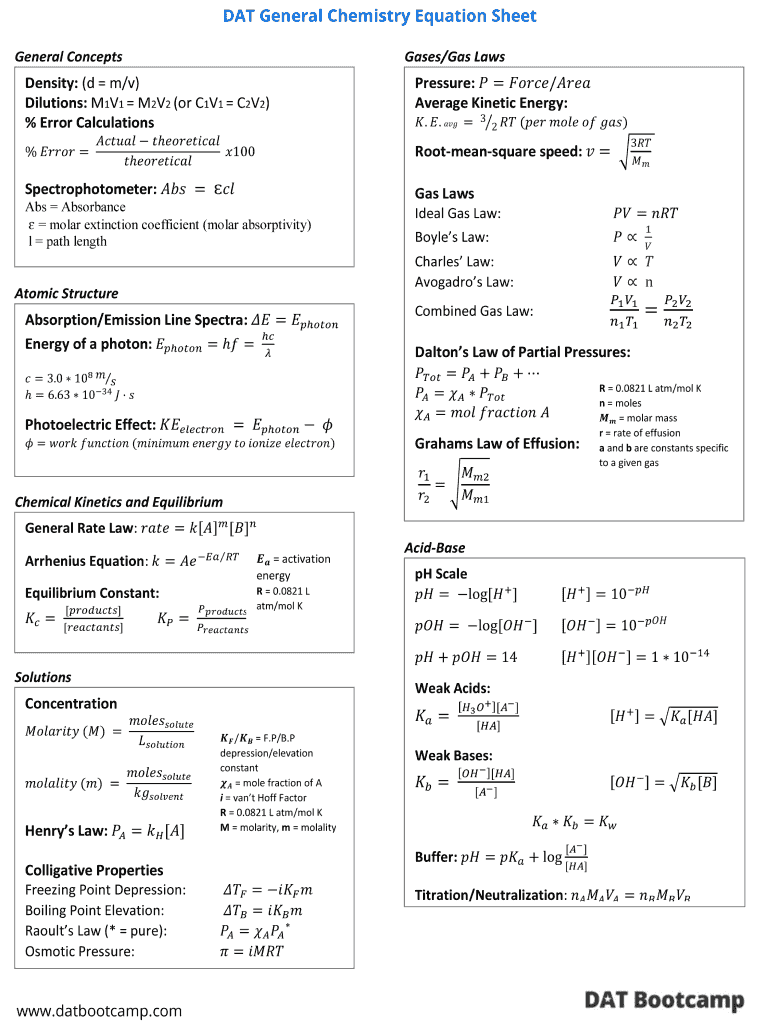
Chemistry Formula Sheet Pdf Fill Online, Printable, Fillable, Blank
Chapter 7 Chemical Formulas And Chemical Compounds LarsDamians
Chapter 7 Test Review
PPT Chapter 7 Chemical Formulas and Chemical Compounds PowerPoint
11+ Chapter 7 Review Chemical Formulas And Chemical Compounds Answer
Chapter 7 Chemical Formulas and Chemical Compounds
PPT Chapter 7 Chemical Formulas and Chemical Compounds PowerPoint
PPT Chapter 7 Chemical Formulas & Compounds PowerPoint Presentation
Related Post: