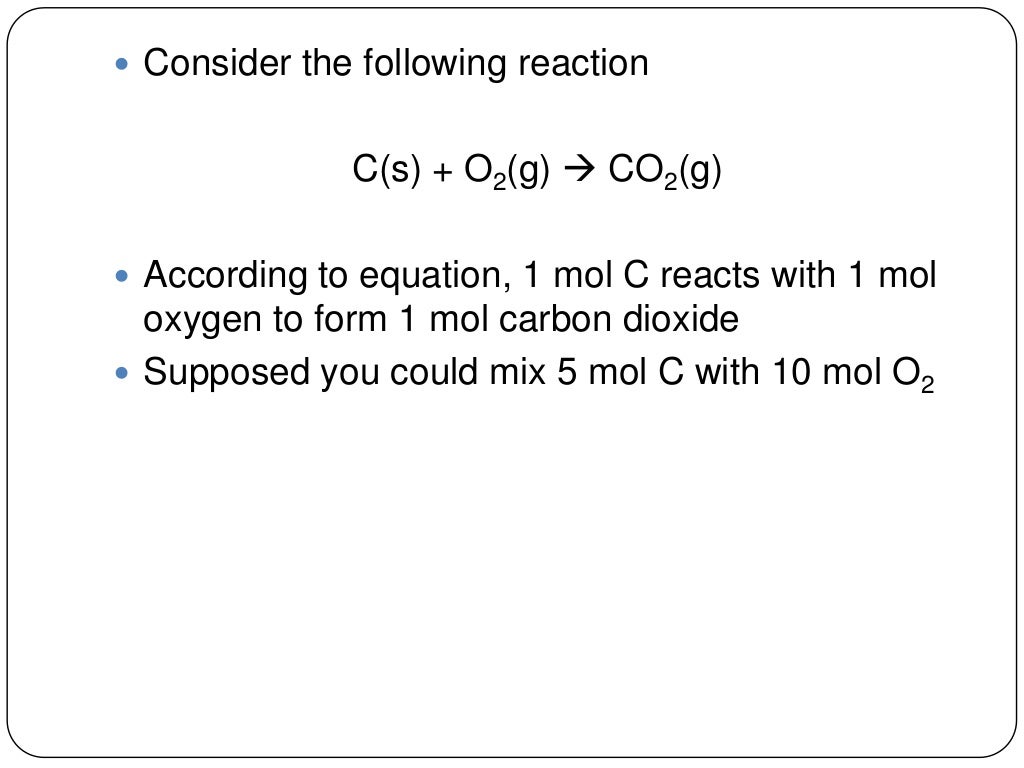
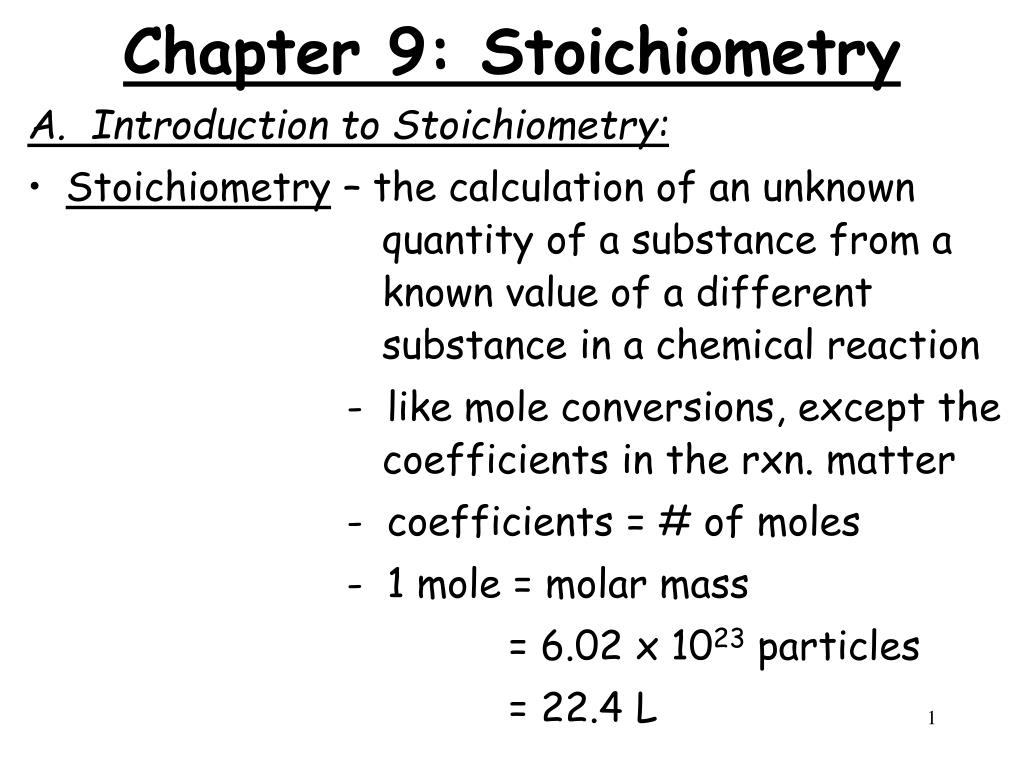
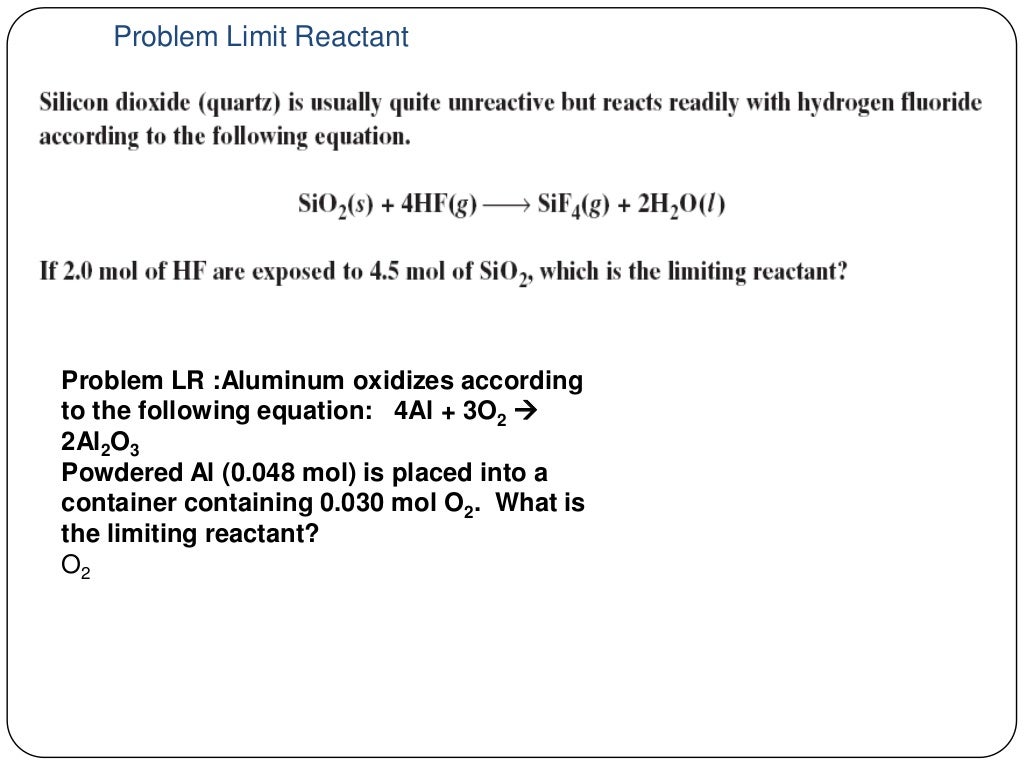
Chapter 9 Review Stoichiometry
Chapter 9 Review Stoichiometry - Stoichiometry quiz for 10th grade students. Web step 1) write a balanced equation. (a)the mole ratio of al to cl in the compound aluminum chloride. Click the card to flip 👆. Click the card to flip 👆. 1 mole = ____ liters. _____ the coefficients in a chemical equation represent the (a) masses in grams of all reactants. Deals with the mass relationship of elements in compounds. Stoichiometry is the branch of chemistry that deals with elements in compounds and with reactants and products in chemical. • calculate the amount of reactants required, or product formed, in a nonchemical process. Web masses, in grams, of all reactants and products. Web which of the following would not be studied within the topic of stoichiometry? Which of the following would not be studied in. The amount of energy required to break the ionic bonds in calcium fluoride. Web from a general summary to chapter summaries to explanations of famous quotes, the sparknotes. Web short answer answer the following questions in the space provided. _____ the coefficients in a chemical equation represent the (a) masses in grams of all reactants. All stoichiometry problems must start with a. Explain the concept of mole ratio as used in reaction stoichiometry problems. 1 mole = ____ liters. Stoichiometry review and chapter summary. Relative numbers of moles of reactants and products. Web masses, in grams, of all reactants and products. Web chapter 9 test : Click the card to flip 👆. Web short answer answer the following questions in the space provided. Which of the following would not be studied in. Web chapter 9 test : Click the card to flip 👆. Web chemistry chapter 9 review: (b)the mass of carbon produced. All stoichiometry problems must start with a. Web for the basics of stoichiometry, review this chapter (excluding the stoichiometry applications, as we will be looking at those in more detail later in this chapter.) for. Web chemistry chapter 9 review: Stoichiometry review and chapter summary. Web 1 mole = ____ molecules or atoms. Stoichiometry quiz for 10th grade students. Explain the concept of mole ratio as used in reaction stoichiometry problems. Web section 9.1 the arithmetic of equations. Given and unknown quantities are amounts in. What is the source of this ratio. Step 3) write the needed mole ratio. Web from a general summary to chapter summaries to explanations of famous quotes, the sparknotes review of stoichiometry study guide has everything you need to ace. Step 4) calculate the molar mass of the given quantity. _____ the coefficients in a chemical equation represent the (a). Step 3) write the needed mole ratio. Web teacher terms in this set (19) in the formation of carbon dioxide from carbon monoxide and oxygen, how many moles of carbon monoxide are needed to react completely with 7.0. • calculate the amount of reactants required, or product formed, in a nonchemical process. Given and unknown quantities are amounts in. 1. A balanced chemical equation allows one to determine the _____ _____ between all compounds in the equation. Web terms in this set (9) composition stoichiometry. Web short answer answer the following questions in the space provided. What is the source of this ratio. 1 mole = ____ liters. Click the card to flip 👆. What pollutant forms when automobile emissions react with oxygen gas and ultraviolet rays. Web from a general summary to chapter summaries to explanations of famous quotes, the sparknotes review of stoichiometry study guide has everything you need to ace. What is the source of this ratio. Step 3) write the needed mole ratio. Web chapter 9 test : Web teacher terms in this set (19) in the formation of carbon dioxide from carbon monoxide and oxygen, how many moles of carbon monoxide are needed to react completely with 7.0. Web chapter 9 stoichiometry test review sheet. Molar mass is calculated from the. Stoichiometry quiz for 10th grade students. Web from a general summary to chapter summaries to explanations of famous quotes, the sparknotes review of stoichiometry study guide has everything you need to ace. Explain the concept of mole ratio as used in reaction stoichiometry problems. _____ the coefficients in a chemical equation represent the (a) masses in grams of all reactants. Web terms in this set (9) composition stoichiometry. Find other quizzes for chemistry and more on quizizz for free! Relative numbers of moles of reactants and products. Click the card to flip 👆. Stoichiometry is the branch of chemistry that deals with elements in compounds and with reactants and products in chemical. (b)the mass of carbon produced. Given and unknown quantities are amounts in. What is the source of this ratio. We can write a mole ratio for a pair. Using ratios from the balanced equation to convert the given quantity. Web short answer answer the following questions in the space provided. (a)the mole ratio of al to cl in the compound aluminum chloride.Chapter 9 Review Stoichiometry Section 2 JiahShaila
PPT Chapter 9 Stoichiometry PowerPoint Presentation, free download
Chapter9 stoichiometry100707061730phpapp01
15+ Chapter 9 Review Stoichiometry Answers With Work AmbreLillyanne
Chapter 9 Review Stoichiometry Section 2 JiahShaila
Name Answer Key Date Chapter 9 Stoichiometry
Practice Test (Ch. 09 Stoichiometry)
Chapter 9 Stoichiometry Chapter Review Answers
PPT Chapter 9 Stoichiometry PowerPoint Presentation, free download
Chapter9 stoichiometry100707061730phpapp01
Related Post: