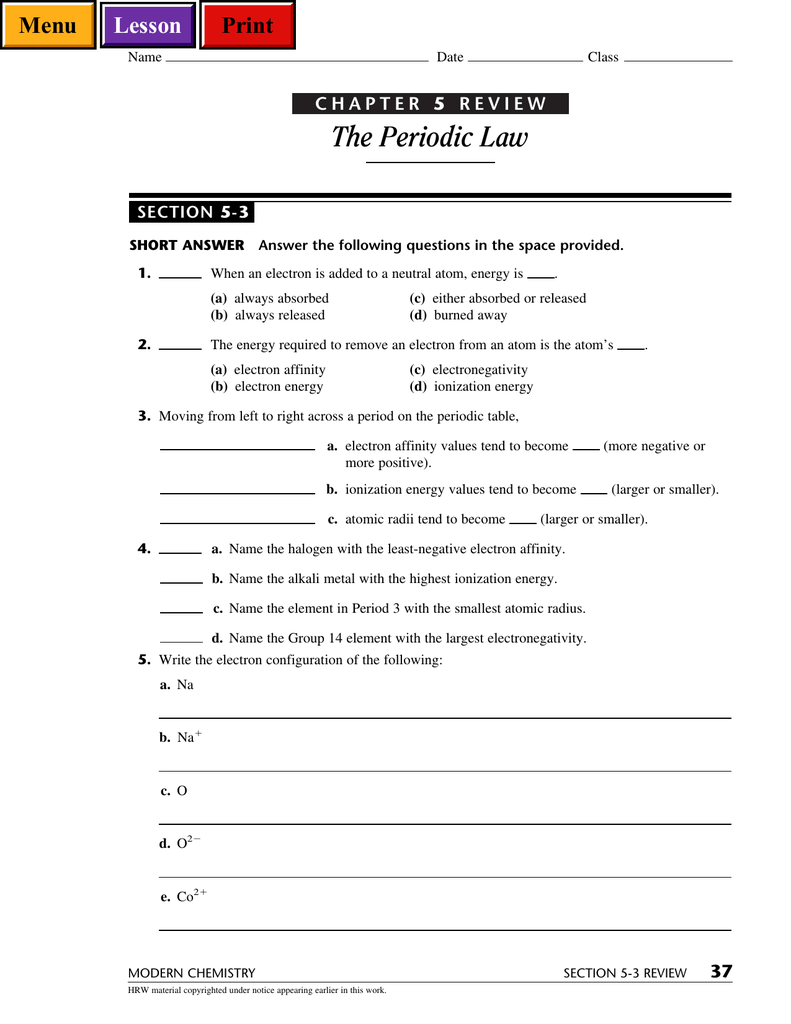
Chapter 5 Review The Periodic Law
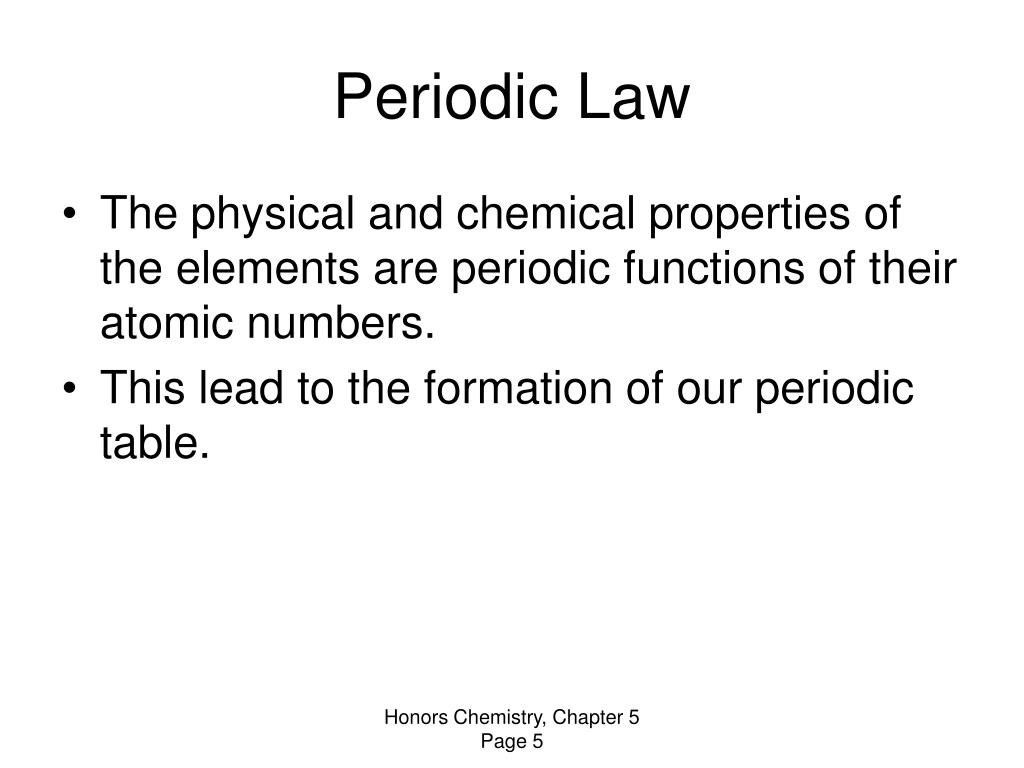
Chapter 5 Review The Periodic Law - Web study with quizlet and memorize flashcards containing terms like the table was developed by john newlands and is based on a relationship called the law of, according to this law, the properties of the elements repeated every, thus, for example, elements two and element _____ have similar properties and more. The periodic law states that the properties of elements are periodic functions of their atomic numbers. The modern periodic law states that. An arrangement of elements in order if their atomic numbers so that the elements with similar properties fall in the same column. This includes only electrons in the s and p orbitals. A measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound. Compare the periodic trends of atomic radii, ionization energy, and electronegativity, and state the reasons for these variations. (b) the physical and chemical properties of an element are functions of its atomic number. The periodic law 5.0 (3 reviews) mendeleev click the card to flip 👆 who noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals? Write the electron configuration of the following: Identify the electron that is removed in the third ionization energy of mg. The physical and chemical properties of an element are function in the same place in an atom. Define atomic and ionic radii, ionization energy, electron affinity, and electronegativity. Na identify the electron that is removed in the first ionization energy of mg. The modern periodic law states. The discovery of the noble gases changed mendeleev's periodic table by adding a new. (b) the physical and chemical properties of an element are functions of its atomic number. Web the modern periodic law states that (a) no two electrons with the same spin can be found in the same place in an atom. Define atomic and ionic radii, ionization. (c) electrons exhibit properties of both particles and waves. An arrangement of elements in order if their atomic numbers so that the elements with similar properties fall in the same column. This means that the _____ determines the position of each element in the periodic table. Compare the periodic trends of atomic radii, ionization energy, and electronegativity, and state the. Web the modern periodic law states that. Compare the periodic trends of atomic radii, ionization energy, and electronegativity, and state the reasons for these variations. The physical and chemical properties of an element are function in the same place in an atom. The electrons available to be lost, gained, or shared in the formation of chemical compounds. Web study with. Define atomic and ionic radii, ionization energy, electron affinity, and electronegativity. The electrons available to be lost, gained, or shared in the formation of chemical compounds. The discovery of the noble gases changed mendeleev's periodic table by adding a new. (b) the physical and chemical properties of an element are functions of its atomic number. The discovery of the noble. A measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound. The periodic law 5.0 (3 reviews) mendeleev click the card to flip 👆 who noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals? The electrons. Web the modern periodic law states that (a) no two electrons with the same spin can be found in the same place in an atom. This includes only electrons in the s and p orbitals. Define atomic and ionic radii, ionization energy, electron affinity, and electronegativity. Identify the electron that is removed in the third ionization energy of mg. Compare. Write the electron configuration of the following: The physical and chemical properties of the elements are periodic functions of their atomic numbers. Define atomic and ionic radii, ionization energy, electron affinity, and electronegativity. The most distinctive property of the noble gases is that they are. Web the modern periodic law states that (a) no two electrons with the same spin. The discovery of the noble gases changed mendeleev's periodic table by adding a new. A measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound. The most distinctive property of the noble gases is that they are. The periodic law 5.0 (3 reviews) mendeleev click the card to flip 👆. This includes only electrons in the s and p orbitals. The discovery of the noble gases changed mendeleev's periodic table by adding a new. An arrangement of elements in order if their atomic numbers so that the elements with similar properties fall in the same column. Web the modern periodic law states that. The physical and chemical properties of the. A measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound. Web the modern periodic law states that (a) no two electrons with the same spin can be found in the same place in an atom. Na identify the electron that is removed in the first ionization energy of mg. The electrons available to be lost, gained, or shared in the formation of chemical compounds. The discovery of the noble gases changed mendeleev's periodic table by adding a new. The physical and chemical properties of an element are function in the same place in an atom. Web a negatively charged ion. Define atomic and ionic radii, ionization energy, electron affinity, and electronegativity. The periodic law states that the properties of elements are periodic functions of their atomic numbers. Web in the modern periodic table, elements are ordered according to. An arrangement of elements in order if their atomic numbers so that the elements with similar properties fall in the same column. Compare the periodic trends of atomic radii, ionization energy, and electronegativity, and state the reasons for these variations. The most distinctive property of the noble gases is that they are. The periodic law 5.0 (3 reviews) mendeleev click the card to flip 👆 who noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals? (b) the physical and chemical properties of an element are functions of its atomic number. Identify the electron that is removed in the second ionization energy of mg. Identify the electron that is removed in the third ionization energy of mg. The most distinctive property of the noble gases is that they are. Web the modern periodic law states that. The physical and chemical properties of the elements are periodic functions of their atomic numbers.PPT Chapter 5 Periodic Law PowerPoint Presentation, free download
PPT Chapter 5 Periodic Law PowerPoint Presentation, free download
PPT Chapter 5 The Periodic Law PowerPoint Presentation, free
ch5studyguide
17+ Chapter 5 The Periodic Law RajinderRowan
The Periodic Law Menu Print Lesson
PPT Chapter 5 The Periodic Law PowerPoint Presentation, free download
PPT Modern Chemistry Chapter 5 The Periodic Law PowerPoint
PPT Chapter 5 Periodic Law PowerPoint Presentation, free download
PPT Chapter 5 The Periodic Law PowerPoint Presentation, free download
Related Post: